Calcium Clock
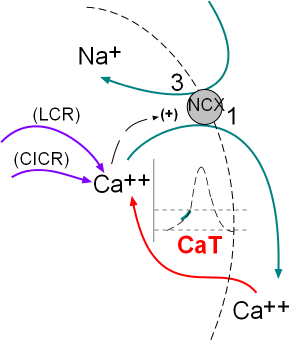 The activity of the NCX exchangers is also activated by calcium ion release from the sarcoplasmic reticulum (SR). The initial
release is called local calcium release (LCR) and occurs simultaneously with the influx of calcium through the CaT channels.
The second release called calcium-induced calcium release (CICR) is during depolarization; this represents a 'tick' of the calcium clock.
The illustration at the left indicates these activities.
The activity of the NCX exchangers is also activated by calcium ion release from the sarcoplasmic reticulum (SR). The initial
release is called local calcium release (LCR) and occurs simultaneously with the influx of calcium through the CaT channels.
The second release called calcium-induced calcium release (CICR) is during depolarization; this represents a 'tick' of the calcium clock.
The illustration at the left indicates these activities.
The previous series of tutorials described the structure and function of the various membrane-bound channels and exchangers that
produce the pacemaker action potential. That series of voltage-regulated activities has been named the 'membrane clock'.
Research during the past few
decades has verified that the cyclical release of calcium from the sarcoplasmic reticulum, the 'calcium clock', also plays an
important role in modifying this action potential.
Sarcoplasmic Reticulum
The histology of pacemaker cells is quite different from that of myocardial contractile cells. Pacemaker cells are spindle or stellate shaped
and extremely small with a diameter of only 4nm. They are densely covered with caveloli ... small flask-shaped indentations ... that accounts
for 50% of their surface area. They have few and unorganized myofilaments, numerous mitochondria and a sarcoplasmic reticulum (SR) found
throughout the cytoplasm. The sections of SR that abut the sarcolemma (cell membrane) are referred to as 'junctional' while the remaining sections are
referred to as 'network'.
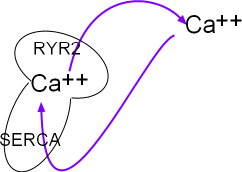
The portion of the main diagram at the right represent the sarcoplasmic reticulum (SR) as a mushroom.
Calcium ions are pumped into it by the activity of sarcoplasmic reticulum
calcium ATPase (SERCA) located in the organelle's network membrane. These ions diffuse toward and accumulate beneath ryanodine
receptor channels (RYR2) located in the junctional membrane. When the RYR2 channels open the ions diffuse into
the cytoplasm just beneath the cell membrane. From there they are either removed by NCX exchangers or returned (purple arrows)
to the lumen of this organelle by SERCAs.
Structure of SR Membrane Components
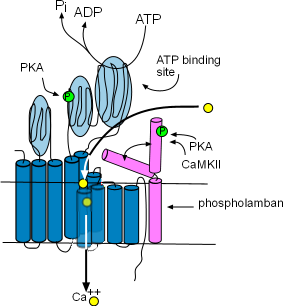
Sarcoplasmic Reticulum Calcium ATPase (SERCA)
These enzymes are bound to the membrane of the SR in the network region and are responsible for refilling the SR lumen.
Using the energy released by ATP, they pump Ca2+ from the
cytoplasm into the lumen (interior) of the SR. The illustration at the right
shows that this enzyme is composed of ten helices (dark blue) and large cytoplasmic loops (light blue). There are binding sites for
phosphokinase A (PKA) to phosphorylate (green 'P' circle) the enzyme and ATP to provide the energy source. The yellow circles show that
Ca2+ is transported into the SR lumen between the helices.
Phospholamban (pink) is a two-helix protein closely associated with SERCA. The illustration shows that it can
assume two conformations, one in which a helix is bent over a portion of SERCA blocking the calcium ion pathway and the other
unbent shape that does not obstruct the pathway. The double arrow represents the conversion of one conformation to the other.
Phosphorylation by Ca2+-calmodulin-dependent protein kinase (CaMKII) and PKA favors the 'open' conformation and
increase the pumping rate filling the lumen faster. The concentration of CaMKII increases during depolarization; the concentration
of PKA is controlled by the autonomic nervous system.
Ryanodine Receptors (RYR2)
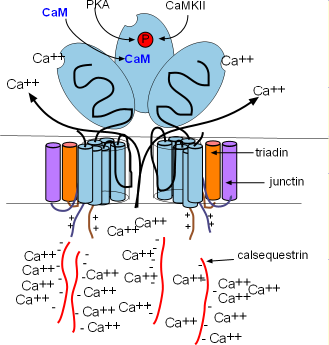 Ryanodine receptors are embedded in the SR membrane at the junctional region that is located just beneath the cell membrane. They
are channels that open to release Ca2+ into the cytoplasm. RYR2s are arranged in groups called
'calcium release units' (CRUs). There are ~10,000 CRUs per cell, with 100 RYRs each, giving a total of ~1,000,000 RYRs per cell.
Ryanodine receptors are embedded in the SR membrane at the junctional region that is located just beneath the cell membrane. They
are channels that open to release Ca2+ into the cytoplasm. RYR2s are arranged in groups called
'calcium release units' (CRUs). There are ~10,000 CRUs per cell, with 100 RYRs each, giving a total of ~1,000,000 RYRs per cell.
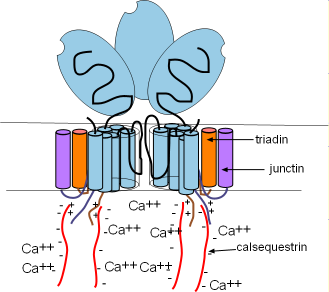
These channels are ~10X larger than most other channels. Each consists of four
separate proteins referred to as 'monomers' (light blue) that are arranged around a pore. Several helices pass through the SR
membrane with a large (light blue oval) cytoplasmic foot containing several binding sites as shown in the right illustration.
There are two other membrane-embedded proteins associated with each monomer; triadin (orange) and junctin (purple). Each
consists of one alpha helix and a positively-charged C terminal extending into the cytoplasm (pairs of short wavy lines). Within
the lumen of the SR, negatively-charged calsequestrin (CASQ2) molecules (long wavy red lines) bond to these positive sites as shown
in the illustration. These groupings are called a calcium-sensing receptors.
Control Mechanisms
The calcium-sensing receptors cause the channels to open when luminal Ca2+ concentrations increase (intrinsic control).
This occurs when Ca2+ bind to calsequestrin (CASQ2) molecules detaching them from the calcium-sensing receptors as shown
in the right illustration. Calcium released by this mechanism is called a local calcium release (LCR). Extrinsic control occurs when binding sites on the feet open the channels in response to high
concentrations of Ca2+ and PKA in the cytoplasm. Calcium released by this mechanism is called calcium-induced calcium
release (CICR).
.
Intrinsic Control & LCR
 SERCAs pump continually so that
Ca2+ is always filling the SR and diffusing toward the RYR2s in junctional regions.
As more and more Ca2+ attaches to the calcium-sensing receptors the CASQ2 molecules
will detach and open the channel (right illustration above). This causes a local calcium release (LCR) from the SR.
This loss of luminal Ca2+ is restored as Ca2+
unbinds from CASQ2s allowing them to reattach to the RYR2s and close the channels (left illustration above).
SERCAs pump continually so that
Ca2+ is always filling the SR and diffusing toward the RYR2s in junctional regions.
As more and more Ca2+ attaches to the calcium-sensing receptors the CASQ2 molecules
will detach and open the channel (right illustration above). This causes a local calcium release (LCR) from the SR.
This loss of luminal Ca2+ is restored as Ca2+
unbinds from CASQ2s allowing them to reattach to the RYR2s and close the channels (left illustration above).
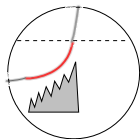
Individual LCRs occur rapidly, each starting before the preceding one has ended, causing the stair-step pattern
shown in the illustration at the left. This is thought to occur because binding of LCR Ca2+ to the
RYR2 feet makes them more likely to respond to internal Ca2+ supplies.
Extrinsic Control & CICR
Calcium-induced calcium release (CICR) occurs when the influx of Ca2+ through CaL channels
attaches to RYR2 binding sites on RYR2 'feet' holding those channels open until ~66% of the remaining Ca2+
in the SR has been released. The channels close when sufficient cytoplasmic Ca2+ have been removed by (1) the NCX exchangers
(2) SERCA or, (3) membrane-bound calcium pumps. The activity levels of each of these depends on the concentration of Ca2+
in the cytoplasm.
Coupled Clocks
The intrinsic control mechanism of the sarcoplasmic reticulum causes the release of 'spurts' of Ca2+ (LCRs) that
increase the activation of membrane-bound NCX exchangers. Additionally, the rapid influx of Ca2+ through the
membrane-bound CaL channels opens the RYR2 channels of the SR causing a second Ca2+ release (CICR). In this manner
the activities of membrane-bound components (membrane clock) and activities of reticulum-bound components (calcium clock) interact;
the clocks are doubly coupled together.
Updated:1/30/2016
Continue to Autonomic Nervous System
Return to previous tutorial... Late Diastolic Depolarization
Return to home page
 The activity of the NCX exchangers is also activated by calcium ion release from the sarcoplasmic reticulum (SR). The initial
release is called local calcium release (LCR) and occurs simultaneously with the influx of calcium through the CaT channels.
The second release called calcium-induced calcium release (CICR) is during depolarization; this represents a 'tick' of the calcium clock.
The illustration at the left indicates these activities.
The activity of the NCX exchangers is also activated by calcium ion release from the sarcoplasmic reticulum (SR). The initial
release is called local calcium release (LCR) and occurs simultaneously with the influx of calcium through the CaT channels.
The second release called calcium-induced calcium release (CICR) is during depolarization; this represents a 'tick' of the calcium clock.
The illustration at the left indicates these activities.


 Ryanodine receptors are embedded in the SR membrane at the junctional region that is located just beneath the cell membrane. They
are channels that open to release Ca2+ into the cytoplasm. RYR2s are arranged in groups called
'calcium release units' (CRUs). There are ~10,000 CRUs per cell, with 100 RYRs each, giving a total of ~1,000,000 RYRs per cell.
Ryanodine receptors are embedded in the SR membrane at the junctional region that is located just beneath the cell membrane. They
are channels that open to release Ca2+ into the cytoplasm. RYR2s are arranged in groups called
'calcium release units' (CRUs). There are ~10,000 CRUs per cell, with 100 RYRs each, giving a total of ~1,000,000 RYRs per cell. SERCAs pump continually so that
Ca2+ is always filling the SR and diffusing toward the RYR2s in junctional regions.
As more and more Ca2+ attaches to the calcium-sensing receptors the CASQ2 molecules
will detach and open the channel (right illustration above). This causes a local calcium release (LCR) from the SR.
This loss of luminal Ca2+ is restored as Ca2+
unbinds from CASQ2s allowing them to reattach to the RYR2s and close the channels (left illustration above).
SERCAs pump continually so that
Ca2+ is always filling the SR and diffusing toward the RYR2s in junctional regions.
As more and more Ca2+ attaches to the calcium-sensing receptors the CASQ2 molecules
will detach and open the channel (right illustration above). This causes a local calcium release (LCR) from the SR.
This loss of luminal Ca2+ is restored as Ca2+
unbinds from CASQ2s allowing them to reattach to the RYR2s and close the channels (left illustration above).