 'Isomerization' is a reaction that alters the structural formula of the molecule but does not
change the chemical formula. No other molecules take part in this type of reaction.
'Isomerization' is a reaction that alters the structural formula of the molecule but does not
change the chemical formula. No other molecules take part in this type of reaction.The term 'glycolysis' means sugar decomposition or breakdown ('glyco-' is from the Greek for sweet --- glucose used to be named glycose). All reactions occur in the cytoplasm. Glycolysis consists of a sequence of reactions grouped as:
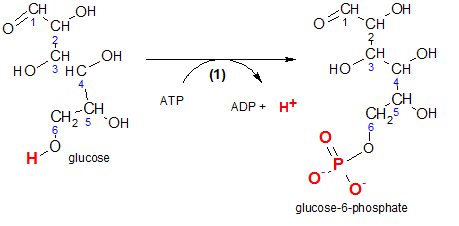
'Phosphorylation' means to add a phosphate group (-PO3-2) to the reactant. Physiologically, this prevents the diffusion of glucose back out of the cell.
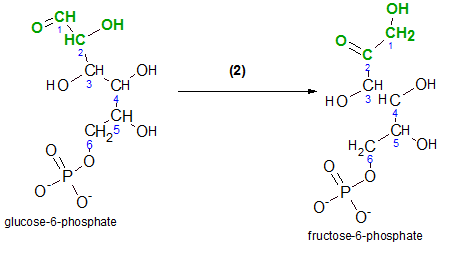
Inspect the reactant, glucose, and notice the carbons in its chain are numbered. This numbering notation is important because it gives meaning to some of the chemical names we will encounter. For example, inspection of the product, glucose-6-phosphate, indicates the phosphate group is associated with carbon #6. Verify the two negative charges on that group. These charges are there because these two hydroxyl groups (-OH) have ionized (-O- and H+).
The enzyme responsible for this reaction is hexokinase. 'Hexo' means 'six' because the reactant is a six-carbon molecule and a 'kinase' is an enzyme that catalyzes the phosphorylation of a reactant by ATP. In other words, it transfers a phosphate group from ATP to another molecule...in this case, glucose. It would be helpful at this point if you would quickly review the details of ATP/ADP reactions by clicking here .
The atoms that are involved are shown in red. Comparison of carbon #6 on both the reactant and the product shows that a phosphate group is now located where there once was only a hydrogen. The hydrogen is now a free hydrogen ion shown with ADP.
 'Isomerization' is a reaction that alters the structural formula of the molecule but does not
change the chemical formula. No other molecules take part in this type of reaction.
'Isomerization' is a reaction that alters the structural formula of the molecule but does not
change the chemical formula. No other molecules take part in this type of reaction.
Begin by comparing the structures of both molecules. The atoms involved in the reaction are shown in green.
If you look closely at the green portions of each molecule you'll notice that the bond between carbons #2 and #3 appears rotated 180 degrees.
The enzyme phosphoglucose isomerase catalyzes this reaction. The ending 'ase' indicates that the molecule is an enzyme and 'isomer' indicates that the molecules involved have the same chemical formulas but different chemical structures. The 'phosphoglucose' part of the enzyme's name identifies the reactant, glucose-6-phosphate.
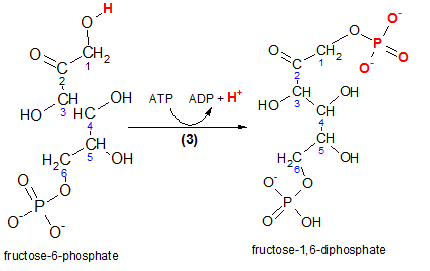 Carefully inspect the reactant, fructose-6-phosphate, in the illustration to the left. The hydrogen (red)
is removed and the terminal phosphate group from ATP takes its place as seen in the product molecule.
The product is named fructose-1,6-diphosphate and you can verify that the two (di)
phosphate groups are associated with carbons #1 and #6. Glance back at the Step 1 reaction and note the
similarity.
Carefully inspect the reactant, fructose-6-phosphate, in the illustration to the left. The hydrogen (red)
is removed and the terminal phosphate group from ATP takes its place as seen in the product molecule.
The product is named fructose-1,6-diphosphate and you can verify that the two (di)
phosphate groups are associated with carbons #1 and #6. Glance back at the Step 1 reaction and note the
similarity.
The enzyme that catalyzes this reaction is phosphofructokinase. Taking the name apart reveals that the reactant, a phosphorylated fructose molecule (phosphofructo), gained another phosphate group from ATP (kinase).
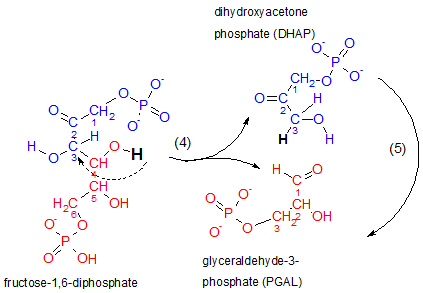 This is the step for which glycolysis is named...'lysis' means 'to separate'. Notice that the reactant
breaks between carbons #3 and #4. The top half of the reactant (blue) becomes dihydroxyacetone phosphate,
DHAP, and the lower half (red) becomes glyceraldehyde-3-phosphate, PGAL. The hydrogen indicated as
black in the reactant loses its association with carbon #4 to become associated with carbon #3 to
break the bond between carbons #3 and #4 (see curved dashed arrow).
This is the step for which glycolysis is named...'lysis' means 'to separate'. Notice that the reactant
breaks between carbons #3 and #4. The top half of the reactant (blue) becomes dihydroxyacetone phosphate,
DHAP, and the lower half (red) becomes glyceraldehyde-3-phosphate, PGAL. The hydrogen indicated as
black in the reactant loses its association with carbon #4 to become associated with carbon #3 to
break the bond between carbons #3 and #4 (see curved dashed arrow).
Inspect the structure of the top product, dihydroxyacetone phosphate. The placement of its double-bonded oxygen classifies it as a ketone. 'Dihydroxy' means 'two hydroxyl (OH-) groups. However, the molecule has only one hydroxyl group on it's #3 carbon...go figure. Acetone (not shown here) is a three-carbon chain with a double-bonded carbon on its #2 carbon and DHAP is similar in this respect thus the inclusion of 'acetone' in this molecule's name. The 'phosphate' part of the name should be obvious.
Inspect the lower product, glyceraldehyde-3-phosphate. It is an aldehyde because the terminal carbon is part of a carbonyl group (-CHO). The double-bonded oxygen of this group is the atom to which the previously mentioned hydrogen atom (black) was attached. The '3' indicates the phosphate is attached to carbon #3. Note the renumbering of carbons in this molecule.
The enzyme responsible for this is aldolase. As always, '-ase' implies the molecule is an enzyme. The 'aldol' part of its name refers to the presence of a double-bonded oxygen with an hydroxyl on an adjacent carbon in the reactant. Verify this by inspecting the atoms attached to carbons #2 and #3 of the reactant.
The enzyme that catalyzes this reaction is triose phosphate isomerase. The curved arrow to the far right in the above illustration indicates that DHAP is internally modified to form PGAL; DHAP and PGAL are isomers as implied by the enzyme's name. 'Triose' means 'a three-carbon sugar'. An easily missed point is that there are now two molecules of PGAL therefore all following reactions will occur twice -- once for each PGAL.
The six-carbon glucose molecule became two (2) three-carbon molecules (PGAL) that have a phosphate group at one end and a carbonyl group (-CHO) on the other. There was a loss of two (2) ATP molecules to accomplish this. There were two phosphorylation, two isomerization and one cleavage reaction.
Continue to Glycolysis II.