Key Concepts
Glycolysis is a series of ten reactions that breaks down glucose into smaller molecules that
enter the cell's mitochondria. It is within these organelles that these glucose remnants are further
decomposed into the waste products carbon dioxide and water. During these later reactions numerous
molecules of ATP are produced. ATP provides energy for the vast majority of the cell's activities.
A point often lost when studying each of these reactions -- and trying to keep track
of the various atoms -- is the importance of electrons and their accompanying hydrogen nuclei (protons). Essentially,
the main theme is that these
molecules are stripped of their electrons. These electrons are destined to take part in a series of
reactions known as the 'electron transport system'. The electrons are usually removes from their substrates
two at a time and accompanied by a matching pair of protons (hydrogen nuclei.)
It is traditional to follow
the course of these electrons by watching their hydrogen nuclei partners and this often leads to quite a bit
of confusion. Therefore, before beginning these tutorials it will be beneficial to get a firm grip on
the various electron/proton arrangements.
Two Types of Hydrogen Ions
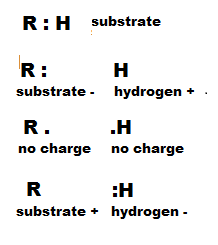 The illustration to the right shows how a hydrogen might be removed from an organic
molecule. The 'R' stands for the substrate molecule -- whatever it might be -- the : is the pair
of electrons that forms a single bond between the substrate and one of its hydrogen atoms. The top picture
illustrates this situation.
The illustration to the right shows how a hydrogen might be removed from an organic
molecule. The 'R' stands for the substrate molecule -- whatever it might be -- the : is the pair
of electrons that forms a single bond between the substrate and one of its hydrogen atoms. The top picture
illustrates this situation.
If the hydrogen is removed -- to 'get at' the electrons bonding it to the substrate --
one of three things might happen:
- The hydrogen nucleus (proton) might be removed leaving both bonding electrons behind. This would result
in a negatively charged substrate and the hydrogen would consist
solely of it's nucleus (one proton). This is an hydrogen ion (H+).
- The hydrogen might be removed taking only one of the two electrons with it. This would result in
both the substrate and the hydrogen being neutral. This is an hydrogen atom (H).
- The hydrogen might be removed taking both bonding electrons with it. This would result in a positively
charged substrate and the hydrogen would have two electrons. This is an hydride ion (H-).
Note that the hydrogen ion carries no electrons. These will become part of a 'pool' of hydrogen ions.
The hydrogen atom carries one electron and you will find it will be 'transported' around by FAD
molecules (see below). The hydride ion carries two electrons and will be 'transported' around by NAD+
(see below).
Oxidation and Reduction
The terms 'oxidation' and 'reduction' are used when there is a transfer of electrons between molecules.
The derivation of these terms is awkward and have been explained in three different ways.
- Oxidation
is the gain of oxidation and reduction is the loss of oxygen.
- Oxidation is the loss of hydrogen and
reduction is the gain of hydrogen.
- Oxidation is the loss of electrons and reduction is the gain of
electrons.
Hint: OIL = Oxidation Is Loss & RIG = Reduction Is Gain.
If there is
an electron loser then there has to be an electron gainer -- oxidation and reduction occur together.
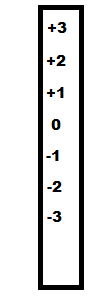 Consider the number scale to the left. If a molecule has a +2 charge then gains an electron its charge will
be reduced to +1 on the scale. This is why the term 'reduction' is defined as the gain of an electron -- RIG.
Because oxidation and reduction
are the opposite of each other, oxidation must be the loss of an electron -- OIL.
Consider the number scale to the left. If a molecule has a +2 charge then gains an electron its charge will
be reduced to +1 on the scale. This is why the term 'reduction' is defined as the gain of an electron -- RIG.
Because oxidation and reduction
are the opposite of each other, oxidation must be the loss of an electron -- OIL.
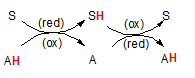 The illustration to the right shows how reduction and oxidation occur together.
The 'S' represents a substrate and 'A' represents another molecule we will call an 'agent'. The 'H' is an
hydrogen atom attached to these molecules. The hydrogen is colored red to emphasize that this
is the atom to keep your eye upon.
The illustration to the right shows how reduction and oxidation occur together.
The 'S' represents a substrate and 'A' represents another molecule we will call an 'agent'. The 'H' is an
hydrogen atom attached to these molecules. The hydrogen is colored red to emphasize that this
is the atom to keep your eye upon.
The pairs of curved arrows coming together then separating mean the two reactants (S and A) come together
then separate. When together the H moves from one to the other. The second pair of curved arrows means
they can come together again and transfer the H back again. The first reaction shows the H on the agent (A) meaning
it is the source of the H -- and it's electron(s). Because A currently holds the electron(s) it is called a
'reducing agent'. Upon giving these electron(s) to the substrate (S), the substrate has now become reduced but
this leaves the agent 'oxidized'. Note the abbreviations (red) and (ox) on the curved arrows. To repeat,
the substrate has become reduced while the reducing agent has become oxidized. Follow this same logic
in the second reaction. In that case the agent is called the 'oxidizing agent' because it causes the other
molecule to become oxidized.
Do not leave this section until you thoroughly understand and can use the terms oxidation, reduction,
oxidizing agent and reducing agent.
Transporters / Carriers
Remember, an electron is lost from a substrate along with an hydrogen nucleus. Only a hydride ion (H-) and
a hydrogen atom (H) carry electrons; the hydride ion carries two while the hydrogen atom carries one.
There are two specific transporter that react with these electron-carrying hydrogen nuclei.
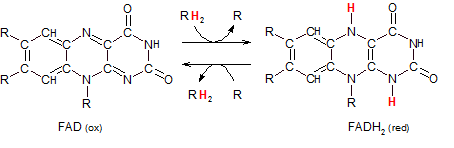
Flavin adenine dinucleotide exists in an oxidized and reduced form.
The RH2 entering the top reaction arrow indicates a pair of hydrogen atoms being
donated from a substrate (R). Because the substrate is always the 'main player' in reactions it will
not be referred to as an 'agent'. It is the other molecule it interacts with that is called an 'agent.'
The intersection of the top two arrows represents the electron-carrying substrate coming into contact
with a transporter (FAD). The pair of hydrogen atoms is transferred from one to the other. The 'main player', R,
has become oxidized (OIL) so the transporter (FAD) must be the oxidizing agent in this reaction. It becomes
reduced to FADH2.
The intersection of the bottom two arrows represents the reaction in reverse. The substrate, R, enters
the reaction in it's oxidized (having previously lost electrons) state. The transporter is in it's reduced form FADH2.
On contact the hydrogen atoms are transferred from one to the other. The 'main player', R, has gained electrons
(RIG) and become reduced while the molecule that 'caused this' -- the reducing agent -- has become oxidized.
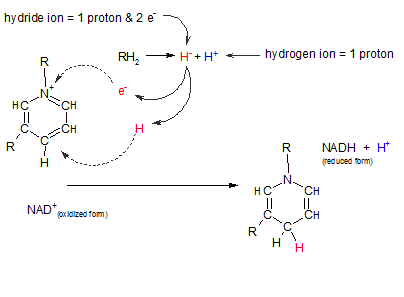
Nicotinamide adenine dinucleotide also exists in two forms: oxidized and reduced. Only the reactive portion of this molecule
is illustrated at the right. The "R" region indicates where the remainder of the molecule
is attached and is of no importance relative to this discussion.
Notice the following in the illustration to the right:
- The oxidized form is a positively charged molecule (NAD+) owing to the absence of an electron at
the position indicated by a plus.
- The reaction at top center (RH2 = H- + H+) represents the loss
of two hydrogens from "R". The two electrons are donated
together on one hydrogen nucleus (a hydride ion (H-) and the other is simply an
hydrogen nucleus(H+).
- When binding with the oxidized form of the transporter (NAD+) the hydride ion loses one of
it's two electrons to the positive charge on the transporter (see curved arrow). When this happens the
hydride ion becomes a hydrogen atom (H) -- with it's one remaining electron. This atom binds to the
transporter where shown by the curved arrow.
- The hydrogen ion (H+) from the substrate remains free and exists in the pool of hydrogen ions
.
- Notice the location of the hydrogen atom (red) in the reduced transporter (NADH). Inspect the change in
double and single bonds in the transporter.
In this reaction NAD+ acts as the oxidizing agent; it takes electrons from the substrate.
In so doing, it becomes reduced and can serve as a reducing agent for some other reaction.
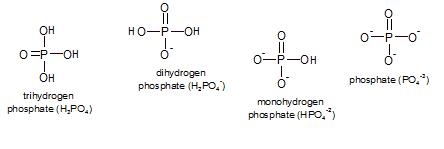
The removal and addition of phosphates are also common types of reactions though they don't necessarily
have to occur hand-in-hand like oxidation and reduction. "Phosphates" are a group of small inorganic (not
containing carbon) molecules that consist of phosphorus (P) with a double-bonded oxygen and three
hydroxyl groups (-OH or -O-). The pH of the environment determines which state(s) will exist and, collectively,
they are referred to as inorganic phosphate (Pi). At physiological conditions a mixture of the
mono- and dihydrogen phosphates are most common.
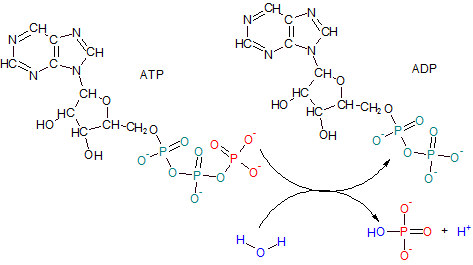
Adenosine Triphosphate (ATP) has a tail of three phosphates--- two teal and one red. ADP results when
the terminal phosphate of ATP (red) is removed by the enzyme ATPase leaving only two phosphates
behind giving the product the name adenosine diphosphate (ADP). The reaction involves the ionization of water
to provide an hydroxyl ion (OH-) (blue) for the released phosphate group and a free hydrogen ion (H+) (blue).
Notice how close together the three double-bonded oxygens are in ATP's phosphate tail. The close proximity of these
oxygens creates a dense area of electrons which repel each other. When the
terminal phosphate is removed the internal "stress" from this charged region is reduced. The potential energy
of the actual bond that was broken to free the phosphate ion is not significantly different from the potential
energy of most other bonds. However, the literature often refers to this bond as 'high-energy'. This leaves the
incorrect impression that more than a normal amount of energy is released when this bond is broken.
Actually, this 'high-energy' reaction refers to the fact that there is significantly less 'overall'
energy (including 'stress') remaining in the molecule -- now ADP -- than when it was ATP.
Continue to Glycolysis I.
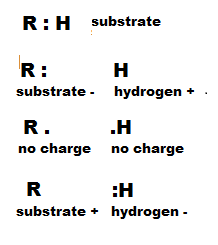 The illustration to the right shows how a hydrogen might be removed from an organic
molecule. The 'R' stands for the substrate molecule -- whatever it might be -- the : is the pair
of electrons that forms a single bond between the substrate and one of its hydrogen atoms. The top picture
illustrates this situation.
The illustration to the right shows how a hydrogen might be removed from an organic
molecule. The 'R' stands for the substrate molecule -- whatever it might be -- the : is the pair
of electrons that forms a single bond between the substrate and one of its hydrogen atoms. The top picture
illustrates this situation. Consider the number scale to the left. If a molecule has a +2 charge then gains an electron its charge will
be reduced to +1 on the scale. This is why the term 'reduction' is defined as the gain of an electron -- RIG.
Because oxidation and reduction
are the opposite of each other, oxidation must be the loss of an electron -- OIL.
Consider the number scale to the left. If a molecule has a +2 charge then gains an electron its charge will
be reduced to +1 on the scale. This is why the term 'reduction' is defined as the gain of an electron -- RIG.
Because oxidation and reduction
are the opposite of each other, oxidation must be the loss of an electron -- OIL.  The illustration to the right shows how reduction and oxidation occur together.
The 'S' represents a substrate and 'A' represents another molecule we will call an 'agent'. The 'H' is an
hydrogen atom attached to these molecules. The hydrogen is colored red to emphasize that this
is the atom to keep your eye upon.
The illustration to the right shows how reduction and oxidation occur together.
The 'S' represents a substrate and 'A' represents another molecule we will call an 'agent'. The 'H' is an
hydrogen atom attached to these molecules. The hydrogen is colored red to emphasize that this
is the atom to keep your eye upon. 


