View Reactions
Intermediate Step & Krebs' Cycle
Krebs' cycle, also called the tricarboxylic acid cycle (TCA), consists of a series of carboxylic
acids. The first such acid is citric acid that has three carboxyl (-COO-) groups. At
physiological conditions these groups exist in this ionic form having lost hydrogen ions to the hydrogen
ion pool. Because this series of acids exists as ions they are named, for example, citrate rather than
citric acid. Throughout the tutorials we will number the carbon chain without including the carboxyl
carbons as part of the numbering scheme.
Breakdown products of all food groups (carbohydrates, fats and proteins) can enter this cycle.
However, most must first be converted into a two-carbon molecule of acetyl coenzyme A (acetyl CoA). This
conversion is called the 'intermediate step' because it links other pathways to Krebs' Cycle. We begin here with
the glucose (a carbohydrate) remnant pyruvate.
The two pyruvates produced at the end of glycolysis
cannot cross the inner membrane. There is an transport system embedded within
the inner mitochondrial membrane that exchanges one hydroxyl ion from the matrix with one pyruvate ion
from the intermembrane space (IMS). Since both pyruvate and hydroxyl ion carry a single negative charge there
is no charge (voltage) change across the membrane due to this activity.
Remember that there are two pyruvates produced from
each glucose thus, as in glycolysis II, both the intermediate step and the cycle occur twice.
Intermediate Step
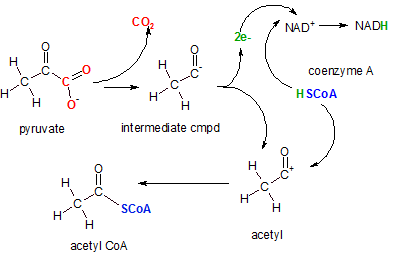 The enzyme pyruvate
decarboxylase catalyzes this complex reaction. The first event is the decarboxylation (removal of a
carboxyl (-COO-) group) to form carbon dioxide (red). The intermediate compound
then donates two electrons (green) to NAD+ to reduce it to NAD- (not shown). Then an
hydrogen ion is donated by coenzyme A complete the formation of NADH. The coenzyme A ion (blue) then attaches
to the positive charge left on the acetyl ion forming acetyl CoA.
The enzyme pyruvate
decarboxylase catalyzes this complex reaction. The first event is the decarboxylation (removal of a
carboxyl (-COO-) group) to form carbon dioxide (red). The intermediate compound
then donates two electrons (green) to NAD+ to reduce it to NAD- (not shown). Then an
hydrogen ion is donated by coenzyme A complete the formation of NADH. The coenzyme A ion (blue) then attaches
to the positive charge left on the acetyl ion forming acetyl CoA.
Step 1 ... Synthesis
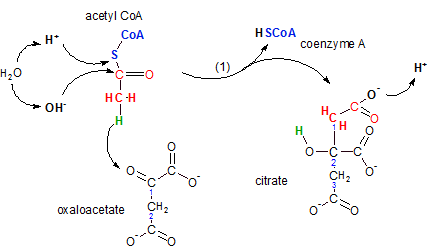 The enzyme citrate
synthase catalyzes the joining of the two-carbon acetyl CoA with the four-carbon oxaloacetate to
form the six-carbon citrate molecule. During this reaction a molecule of coenzyme A is regenerated --
to participate in another occurrence of the previous step.
The enzyme citrate
synthase catalyzes the joining of the two-carbon acetyl CoA with the four-carbon oxaloacetate to
form the six-carbon citrate molecule. During this reaction a molecule of coenzyme A is regenerated --
to participate in another occurrence of the previous step.
Inspect the illustration noting the following:
- Water ionizes
- Acetly CoA is a two-carbon molecule with coenzyme A attached (blue) -- the S (sulfur) is part of the coenzyme
- Oxaloacetate is a two-carbon chain with carboxyl groups (-COO-) attached to carbons #1 and #2 -- it is a four-carbon dicarboxylic acid
- Coenzyme A, when free and not attached to another molecule, has a hydrogen atom attached to it's sulfur.
- Citrate is a three-carbon chain with carboxyl groups attached to each of these three carbons -- it is a six-carbon tricarboxylic acid.
The first event is when the hydrogen (green) on acetyl CoA moves (see curved arrow) to the
oxaloacetate's double-bonded oxygen on carbon #1. Verify its new location on the product citrate; it is
now part of an hydroxyl group (-OH) on citrate's carbon #2. This move opened a bonding site on acetyl CoA's
carbon as well as on carbon #1 of oxaloacetate causing these two bonding sites to combine thus joining the two
reactants together.
Confirm that the two carbon atoms (red) in citrate constitute the acetyl part of the new molecule.
The synthesis of this six-carbon molecule from a two- and a four-carbon molecule required renumbering of the
carbon chain. Notice there are only three carbons in the chain and there are carboxyl groups attached to each
of these.
The upper left of the illustration shows the release of the coenzyme occurs when the bond between
the sulfur and carbon atoms in the acetyl portion is broken. A water molecule is ionized with it's hydrogen
ion bonding to the sulfur and the hydroxyl ion bonding to the carbon. This event releases coenzyme A and also
converts the carbon of the acetyl portion into a carboxyl group. Notice that the hydrogen ion is released
to join the hydrogen ion pool.
Step 2 ... Isomerization
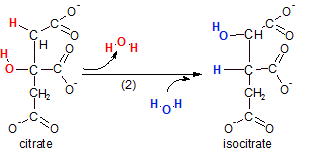 The enzyme aconitase
catalyzes this reaction. The reaction actually takes place in two distinct events. Notice that a water
molecule (red) is removed (dehydration) then a water (blue) is added back. However, the positions of the
hydrogen and hydroxyl groups are now altered. The intermediate compound (not shown) was called 'aconitate'.
It is from this transient compound that the enzyme gets it's name. The overall reaction is an isomerization.
Verify this by counting the atoms in the reactant (citrate) and the substrate (isocitrate) --- note the type
of reaction in the product's name
The enzyme aconitase
catalyzes this reaction. The reaction actually takes place in two distinct events. Notice that a water
molecule (red) is removed (dehydration) then a water (blue) is added back. However, the positions of the
hydrogen and hydroxyl groups are now altered. The intermediate compound (not shown) was called 'aconitate'.
It is from this transient compound that the enzyme gets it's name. The overall reaction is an isomerization.
Verify this by counting the atoms in the reactant (citrate) and the substrate (isocitrate) --- note the type
of reaction in the product's name
Step 3 ...Redox & Decarboxylation
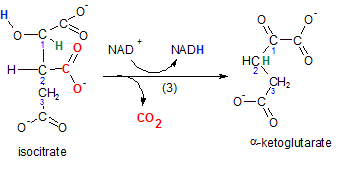 The enzyme for this reaction
is isocitrate dehydrogenase because the reactant, isocitrate, loses hydrogen (blue). Isocitrate is oxidized (OIL) while it's oxidizing agent (NAD+) becomes reduced (RIG) to NADH. As always,
when the oxidizing agent is NAD+, the reactant loses a pair of electrons as an hydride ion
(H-). A second hydrogen (green) is also relocated from carbon #1 to #2. Verify this in the product alpha-ketoglutarate.
The enzyme for this reaction
is isocitrate dehydrogenase because the reactant, isocitrate, loses hydrogen (blue). Isocitrate is oxidized (OIL) while it's oxidizing agent (NAD+) becomes reduced (RIG) to NADH. As always,
when the oxidizing agent is NAD+, the reactant loses a pair of electrons as an hydride ion
(H-). A second hydrogen (green) is also relocated from carbon #1 to #2. Verify this in the product alpha-ketoglutarate.
The relocation of this hydrogen (green) causes the detachment of a carboxyl group (-COO-)
(red). This event is called decarboxylation; carbon dioxide is produced (red).
The product is a three-carbon chain with both a carboxyl and a ketone group associated with
carbon #1. Because the ketone is on carbon #1 -- the alpha carbon --
the product is named alpha-ketoglutarate.
step 4 ...Redox & Decarboxylation
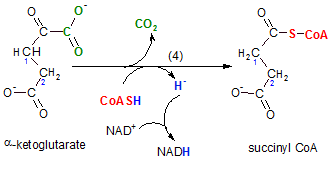 The enzyme for this reaction is alpha-ketoglutarate dehydrogenase. On the surface this reaction
looks like a repeat of the previous step. The two reactions are similar in that:
The enzyme for this reaction is alpha-ketoglutarate dehydrogenase. On the surface this reaction
looks like a repeat of the previous step. The two reactions are similar in that:
- a NAD+ is reduced to NADH, and
- the reactant loses a carboxyl group to produce carbon dioxide.
The difference is that the molecule that is oxidized is coenzyme A instead of the main reactant.
Notice the hydrogen (blue) on coenzyme A is released as a hydride ion (blue). As usual, this is picked up
by the oxidizing agent NAD+ thus reducing it to NADH. When the carboxyl group (green) is removed
the oxidized coenzyme A (red) attaches to that site. The new product, succinyl CoA, is a still a two-carbon
chain but it has only one carbonyl group on carbon #2; there are four carbons in all.
Step 5 ... Synthesis
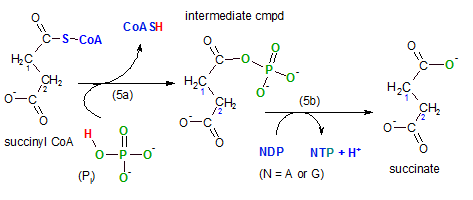 The enzyme for this reaction is succinyl-CoA synthetase; the compound that is being synthesized is
either ATP or GTP (guanidine triphosphate) -- depending on the tissue. In the illustration this is shown
in reaction (5b) where N (nucleotide) is substituted for either A (ATP) or G (GTP). The hydrogen ion (blue) -- entering
the hydrogen ion pool --
is from the NDP to provide a bonding site for the phosphate group (PO3-2) (green) on NTP -- detail
not shown. Observe the fourth oxygen (green) from the original inorganic phosphate (Pi) remains
on the main substrate.
This reaction is a substrate-level phosphorylation reaction as were steps 7 and 10 in glycolysis.
The enzyme for this reaction is succinyl-CoA synthetase; the compound that is being synthesized is
either ATP or GTP (guanidine triphosphate) -- depending on the tissue. In the illustration this is shown
in reaction (5b) where N (nucleotide) is substituted for either A (ATP) or G (GTP). The hydrogen ion (blue) -- entering
the hydrogen ion pool --
is from the NDP to provide a bonding site for the phosphate group (PO3-2) (green) on NTP -- detail
not shown. Observe the fourth oxygen (green) from the original inorganic phosphate (Pi) remains
on the main substrate.
This reaction is a substrate-level phosphorylation reaction as were steps 7 and 10 in glycolysis.
Going back to the first event (5a) in this step we see that the coenzyme A is released from
succinyl-CoA. Inorganic phosphate (Pi or HPO4-2) breaks the sulfur-carbon
giving it's hydrogen (red) to the coenzyme and it's phosphate ion (green) to the substrate. This forms the
intermediate compound shown with the phosphate group where the coenzyme used to be. Notice that the phosphate
group now attached has four oxygen atoms but when it is released in (5b) one of these oxygen atoms remains
behind with the substrate, succinate.
Comparing the reactant, succinyl-CoA, with the product, succinate, shows there is still a four-carbon
molecule with a two-carbon chain having a carbonyl group (-COO-) on each carbon in the chain.
The distinguishing part of this reaction is the formation of a high-energy compound, either ATP or GTP.
Step 6 ... Dehydrogenation
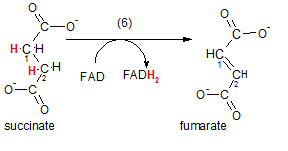 The enzyme is succinic dehydrogenase; it catalyzes the removal of adjacent hydrogen atoms on carbons
#1 and #2 of succinate. The product, fumarate, has a double bond between carbon #1 and #2 as a result. The hydrogen
atoms take electrons with them so this reaction is also classified as an oxidation (OIL); succinate has
been oxidized by the acceptor of the electrons (the oxidizing agent) FAD. As a result, FAD has been reduced
to FADH2.
The enzyme is succinic dehydrogenase; it catalyzes the removal of adjacent hydrogen atoms on carbons
#1 and #2 of succinate. The product, fumarate, has a double bond between carbon #1 and #2 as a result. The hydrogen
atoms take electrons with them so this reaction is also classified as an oxidation (OIL); succinate has
been oxidized by the acceptor of the electrons (the oxidizing agent) FAD. As a result, FAD has been reduced
to FADH2.
This is our first encounter with this transporter and a good time to
review it's structure. This
transporter is unusual in that, instead of floating freely within the mitochondrial matrix, it is attached
to the inner surface of the inner mitochondrial membrane. We will encounter this again in the tutorial
on the electron transport system.
Step 7 ...Hydration
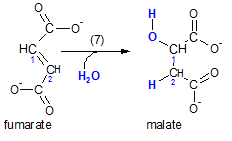 The enzyme fumarase catalyzes this reaction. This is accomplished at the double bond just formed.
A water molecule ionizes with the hydrogen ion going to carbon #2 and the hydroxyl ion to carbon #1.
The enzyme fumarase catalyzes this reaction. This is accomplished at the double bond just formed.
A water molecule ionizes with the hydrogen ion going to carbon #2 and the hydroxyl ion to carbon #1.
Step 8 ...Dehydrogenation
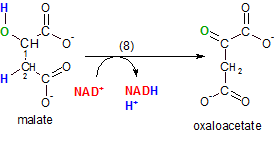 This final Krebs' cycle reaction regenerate the molecule, oxaloacetate, that accepted acetyl CoA into
the cycle in step 1. The enzyme malate dehydrogenase catalyzes the removal of the pair of hydrogen
atoms (blue) placed on carbons #1 and #2 by water in the previous reaction. Notice that the oxygen (green) of
the hydroxyl group is left behind on carbon #1.
This final Krebs' cycle reaction regenerate the molecule, oxaloacetate, that accepted acetyl CoA into
the cycle in step 1. The enzyme malate dehydrogenase catalyzes the removal of the pair of hydrogen
atoms (blue) placed on carbons #1 and #2 by water in the previous reaction. Notice that the oxygen (green) of
the hydroxyl group is left behind on carbon #1.
NAD+ is the oxidizing agent and, as usual, one of the hydrogen atoms is removed as a hydride ion (not
shown) to form NADH and the other hydrogen ion becomes part of the hydrogen ion pool.
Continue to the summary of Krebs' cycle.
 The enzyme pyruvate
decarboxylase catalyzes this complex reaction. The first event is the decarboxylation (removal of a
carboxyl (-COO-) group) to form carbon dioxide (red). The intermediate compound
then donates two electrons (green) to NAD+ to reduce it to NAD- (not shown). Then an
hydrogen ion is donated by coenzyme A complete the formation of NADH. The coenzyme A ion (blue) then attaches
to the positive charge left on the acetyl ion forming acetyl CoA.
The enzyme pyruvate
decarboxylase catalyzes this complex reaction. The first event is the decarboxylation (removal of a
carboxyl (-COO-) group) to form carbon dioxide (red). The intermediate compound
then donates two electrons (green) to NAD+ to reduce it to NAD- (not shown). Then an
hydrogen ion is donated by coenzyme A complete the formation of NADH. The coenzyme A ion (blue) then attaches
to the positive charge left on the acetyl ion forming acetyl CoA. The enzyme citrate
synthase catalyzes the joining of the two-carbon acetyl CoA with the four-carbon oxaloacetate to
form the six-carbon citrate molecule. During this reaction a molecule of coenzyme A is regenerated --
to participate in another occurrence of the previous step.
The enzyme citrate
synthase catalyzes the joining of the two-carbon acetyl CoA with the four-carbon oxaloacetate to
form the six-carbon citrate molecule. During this reaction a molecule of coenzyme A is regenerated --
to participate in another occurrence of the previous step. The enzyme aconitase
catalyzes this reaction. The reaction actually takes place in two distinct events. Notice that a water
molecule (red) is removed (dehydration) then a water (blue) is added back. However, the positions of the
hydrogen and hydroxyl groups are now altered. The intermediate compound (not shown) was called 'aconitate'.
It is from this transient compound that the enzyme gets it's name. The overall reaction is an isomerization.
Verify this by counting the atoms in the reactant (citrate) and the substrate (isocitrate) --- note the type
of reaction in the product's name
The enzyme aconitase
catalyzes this reaction. The reaction actually takes place in two distinct events. Notice that a water
molecule (red) is removed (dehydration) then a water (blue) is added back. However, the positions of the
hydrogen and hydroxyl groups are now altered. The intermediate compound (not shown) was called 'aconitate'.
It is from this transient compound that the enzyme gets it's name. The overall reaction is an isomerization.
Verify this by counting the atoms in the reactant (citrate) and the substrate (isocitrate) --- note the type
of reaction in the product's name  The enzyme for this reaction
is isocitrate dehydrogenase because the reactant, isocitrate, loses hydrogen (blue). Isocitrate is oxidized (OIL) while it's oxidizing agent (NAD+) becomes reduced (RIG) to NADH. As always,
when the oxidizing agent is NAD+, the reactant loses a pair of electrons as an hydride ion
(H-). A second hydrogen (green) is also relocated from carbon #1 to #2. Verify this in the product alpha-ketoglutarate.
The enzyme for this reaction
is isocitrate dehydrogenase because the reactant, isocitrate, loses hydrogen (blue). Isocitrate is oxidized (OIL) while it's oxidizing agent (NAD+) becomes reduced (RIG) to NADH. As always,
when the oxidizing agent is NAD+, the reactant loses a pair of electrons as an hydride ion
(H-). A second hydrogen (green) is also relocated from carbon #1 to #2. Verify this in the product alpha-ketoglutarate. The enzyme for this reaction is alpha-ketoglutarate dehydrogenase. On the surface this reaction
looks like a repeat of the previous step. The two reactions are similar in that:
The enzyme for this reaction is alpha-ketoglutarate dehydrogenase. On the surface this reaction
looks like a repeat of the previous step. The two reactions are similar in that:
 The enzyme for this reaction is succinyl-CoA synthetase; the compound that is being synthesized is
either ATP or GTP (guanidine triphosphate) -- depending on the tissue. In the illustration this is shown
in reaction (5b) where N (nucleotide) is substituted for either A (ATP) or G (GTP). The hydrogen ion (blue) -- entering
the hydrogen ion pool --
is from the NDP to provide a bonding site for the phosphate group (PO3-2) (green) on NTP -- detail
not shown. Observe the fourth oxygen (green) from the original inorganic phosphate (Pi) remains
on the main substrate.
This reaction is a substrate-level phosphorylation reaction as were steps 7 and 10 in glycolysis.
The enzyme for this reaction is succinyl-CoA synthetase; the compound that is being synthesized is
either ATP or GTP (guanidine triphosphate) -- depending on the tissue. In the illustration this is shown
in reaction (5b) where N (nucleotide) is substituted for either A (ATP) or G (GTP). The hydrogen ion (blue) -- entering
the hydrogen ion pool --
is from the NDP to provide a bonding site for the phosphate group (PO3-2) (green) on NTP -- detail
not shown. Observe the fourth oxygen (green) from the original inorganic phosphate (Pi) remains
on the main substrate.
This reaction is a substrate-level phosphorylation reaction as were steps 7 and 10 in glycolysis. The enzyme is succinic dehydrogenase; it catalyzes the removal of adjacent hydrogen atoms on carbons
#1 and #2 of succinate. The product, fumarate, has a double bond between carbon #1 and #2 as a result. The hydrogen
atoms take electrons with them so this reaction is also classified as an oxidation (OIL); succinate has
been oxidized by the acceptor of the electrons (the oxidizing agent) FAD. As a result, FAD has been reduced
to FADH2.
The enzyme is succinic dehydrogenase; it catalyzes the removal of adjacent hydrogen atoms on carbons
#1 and #2 of succinate. The product, fumarate, has a double bond between carbon #1 and #2 as a result. The hydrogen
atoms take electrons with them so this reaction is also classified as an oxidation (OIL); succinate has
been oxidized by the acceptor of the electrons (the oxidizing agent) FAD. As a result, FAD has been reduced
to FADH2.  The enzyme fumarase catalyzes this reaction. This is accomplished at the double bond just formed.
A water molecule ionizes with the hydrogen ion going to carbon #2 and the hydroxyl ion to carbon #1.
The enzyme fumarase catalyzes this reaction. This is accomplished at the double bond just formed.
A water molecule ionizes with the hydrogen ion going to carbon #2 and the hydroxyl ion to carbon #1. This final Krebs' cycle reaction regenerate the molecule, oxaloacetate, that accepted acetyl CoA into
the cycle in step 1. The enzyme malate dehydrogenase catalyzes the removal of the pair of hydrogen
atoms (blue) placed on carbons #1 and #2 by water in the previous reaction. Notice that the oxygen (green) of
the hydroxyl group is left behind on carbon #1.
This final Krebs' cycle reaction regenerate the molecule, oxaloacetate, that accepted acetyl CoA into
the cycle in step 1. The enzyme malate dehydrogenase catalyzes the removal of the pair of hydrogen
atoms (blue) placed on carbons #1 and #2 by water in the previous reaction. Notice that the oxygen (green) of
the hydroxyl group is left behind on carbon #1.