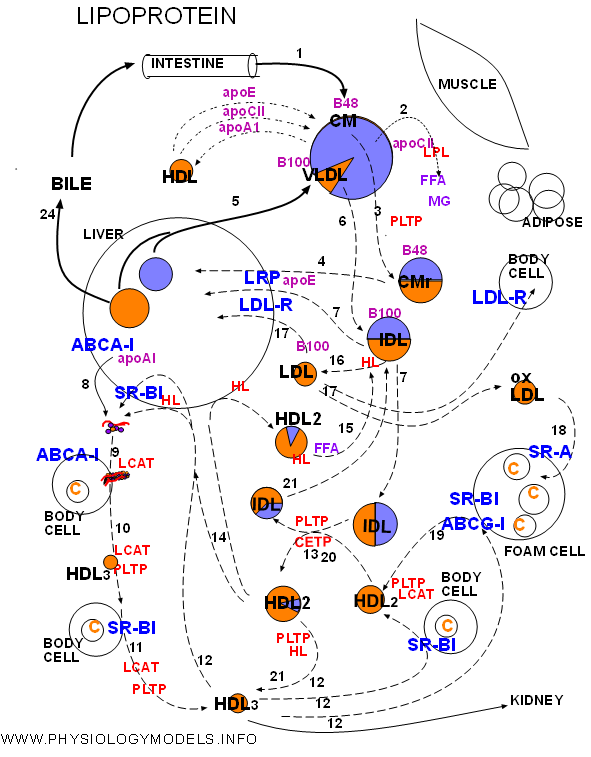
The physiology of lipoproteins will be traced from the entrance of chylomicrons through the return of cholesterol to the liver. Right click on the diagram then choose 'print picture...' from the drop-down menu. You will need to refer to your hard copy throughout this summary.
The numbers on the arrows will allow you to match the diagram with the following narrative.
1. Chylomicrons bring in dietary triglyceride and cholesterol ester from the intestine. These are the largest of the lipoproteins. They have apoprotein 48 on entry but will pick up apoE and apoCII from HDLs and donate apoA1 to them. Triglyceride is by far the most abundant core content.
2. During circulation chylomicrons will encounter lipoprotein lipase (LPL). The inactive enzyme is secreted by muscle and adipose tissue under hormonal control. This enzyme will be activated when bound to apoCII on the chylomicron resulting in rapid hydrolysis of core triglyceride. The products are free fatty acid (FFA) and monoglyceride (MG).
3. Reduction of the core volume reduces the size of the chylomicron forming a remnant. The excess coat material is transferred to phospholipid transfer protein (PLTP).
4. Chylomicron remnants become bound to lipoprotein receptor-related protein (LRP). Apoprotein E, secreted by the liver, is required for binding and the result is phagocytosis.
5. The liver secretes very low density lipoproteins (VLDLs) with approximately the same proportion of core contents as chylomicrons. The triglyceride and cholesterol ester come from intracellular storage sites. Apoprotein B100 is part of the coat.
6. The VLDL, secreted with apoCII as part of its coat, encounters LPL and activates it to hydrolyze its triglyceride. As with chylomicrons there is a reduction in size and loss of excess coat material. The remnant is called intermediate density lipoprotein (IDL).
7. IDL is phagocytized by the liver after, in association with apoE, binding to LRP. It also binds with low density lipoprotein receptor (LDL-R) that recognizes apoB100.
8. High density lipoproteins begin as apoprotein A1 secreted by the liver. The protein ATP-binding cassette A-1 (ABCA-1) transferred excess cholesterol from intracellular storage areas to the hepatocyte membrane. ApoA1 binds with this protein and picks up a few molecules of free cholesterol and phospholipid from the hepatocyte outer leaflet. The disorganized particle called lipid-poor A1 is released into circulation.
9. While circulating, lipid-poor A1 contacts body cells with ABCA-1. Apoprotein A1 of the disorganized particle binds with ABCA-1 resulting in sections of the cells outer leaflet being transferred to the particle. The additional phospholipid and free cholesterol molecules spontaneously form a bi-layered disc-shaped particle called nacent HDL. LCAT on the nacent HDL converts free cholesterol into cholesterol ester that forms a core.
10&11. The curculating small HDL3 accumulates additional cholesterol transferred to the membrane by scavenger receptor B-1 (SR-B1). Contact results in diffusion of cholesterol to HDL. LCAT continues to increase the core. PLTP supplies coat material.
12. HDL3 can follow four paths:
13. Lipoprotein remodeling is exchange of triglyceride and cholesterol ester between lipoproteins along concentration gradients. Particles gaining triglyceride enlarge and those gaining cholesterol ester shrink. CETP and PLTP facilitate this process.
14. Larger HDL2 particles return to the liver where they either deliver cholesterol ester by selective uptake mediated by SR-B1 or are endocytosed after complexing with hepatic lipase and SR-B1 Either way apoA1 is recycled. Alternatively they may liberate hepatic lipase.
15. Hepatic lipase on HDLs is inactive until transferred to triglyceride-rich B100 particles. This occurs after meals (FFA) and the enzyme becomes active.
16. Triglycerides in IDLs are hydrolyzed by hepatic lipase forming LDLs.
17. LDLs can follow three paths:
18. Oxidized LDL is phagocytized by foam cells via SR-A thus facilitating atherosclerosis.
19. Foam cells transport cholesterol to their surface by SR-B1 and ABCG-1 to be picked up by HDL particles.
20. Large lipid exchanges during remodeling would result in triglyceride-rich HDLs and cholesterol ester-rich IDLs.
21. Triglyceride-rich HDLs and cholesterol ester-rich IDLs can be hydrolyzed by hepatic lipase resulting in smaller than usual particles.
Return to home page